Differentiate between ionic bond and covalent bond
Answer:
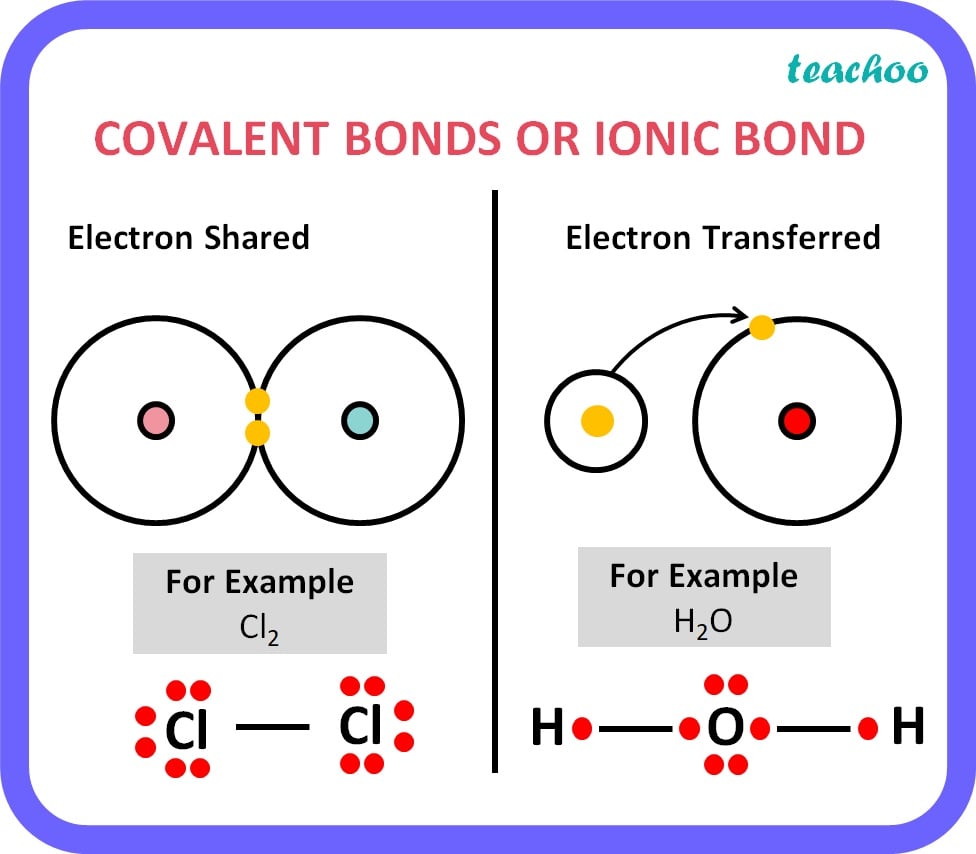
|
Ionic Bond |
Covalent Bond |
|
|
Teachoo Questions
Last updated at Dec. 13, 2024 by Teachoo
Answer:

|
Ionic Bond |
Covalent Bond |
|
|