|
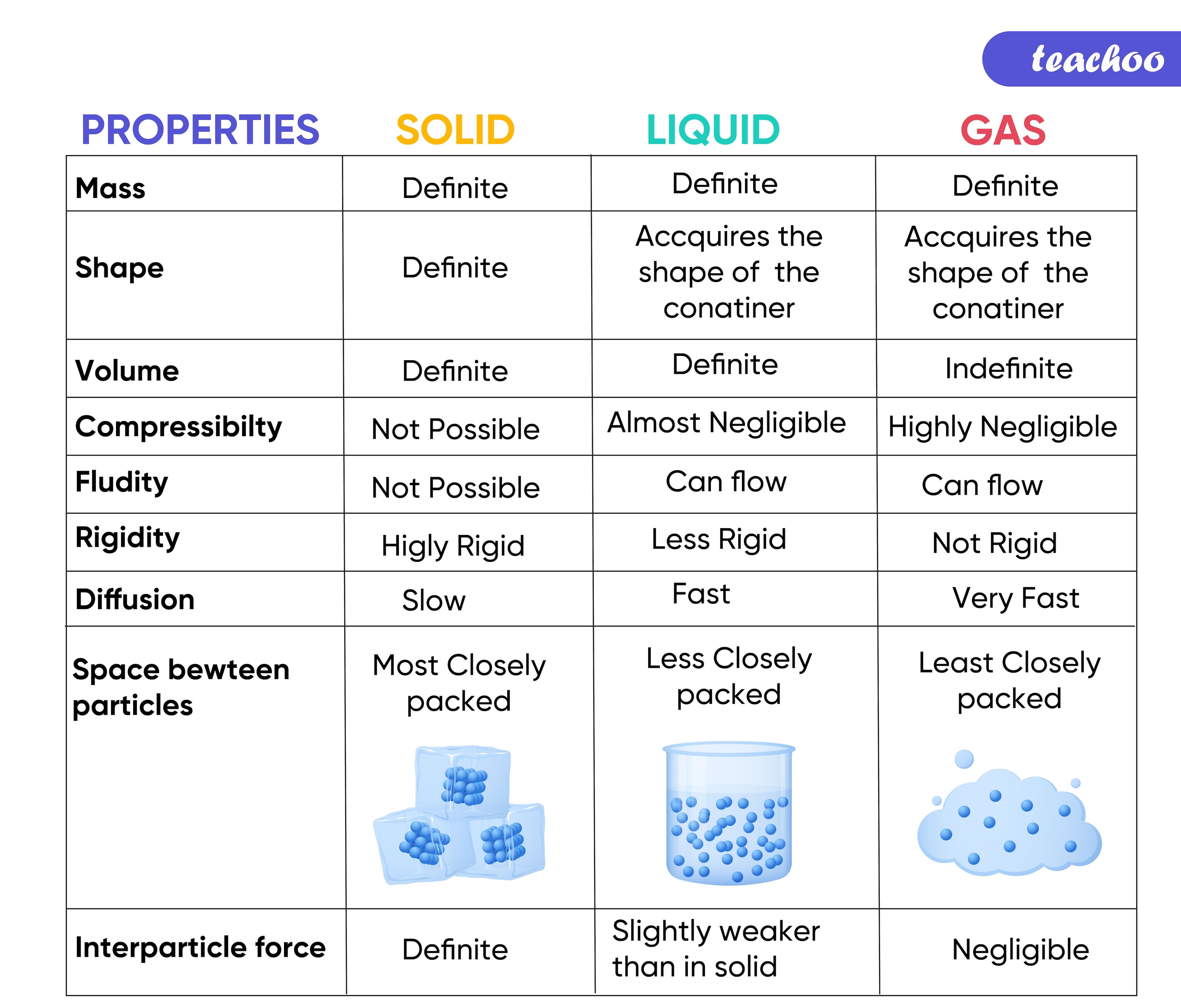
SOLIDS |
LIQUIDS |
GASES |
|
Particles are closely bound |
Particles are close to each other, but not as much as solids |
Particles are much far apart compared to solids and liquids |
|
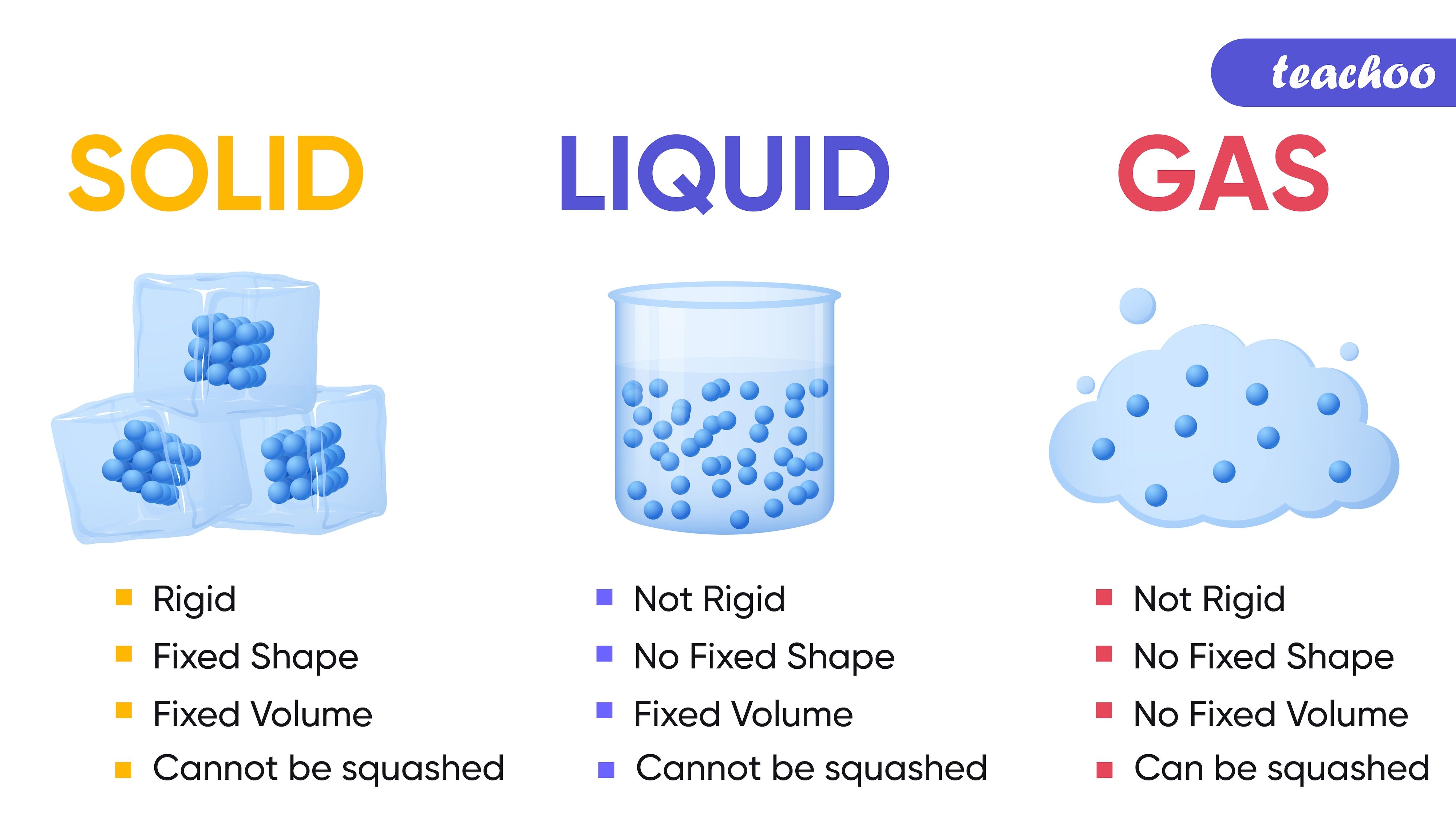
Fixed shape |
No fixed shape, they take the shape of the container |
No fixed shape, fill the entire space they are enclosed in |
|
Fixed volume |
Fixed volume |
No fixed volume |
|
Do not flow like liquids and gases |
Flow easily as particles slide over each other |
Flow easily as the particles can move easily from one place to another |
|
Maintain their form and occupy only as much space as their size |
Do not fill the container completely as they have greater density than gases |
Fill the container fully due to their low density |
|
Cannot be compressed easily |
Cannot be compressed easily as the particles are relatively close to each other |
Can be compressed easily as they have low density |
Let's look at Properties of each one by one
Properties of Solids
-
Solids have fixed shape and fixed volume
Size of solids do not change and it occupies fixed space
-
Particles of Solid are closely bound
There is higher force of attraction between particles
-
Solids do not take shape of container like liquids
Example - If ice is put in a container, it does not take shape of container
-
Solids do not flow like liquids
Liquids flow from one place to another. However, solids have fixed shape and do not flow or move
- Particles of solid cannot be compressed easily
Let's do some questions, shall we?
Question 1
Rubber band changes its shape. Then why is it called solid?
Answer
- Rubber band changes its shape only on stretching (when a force is applied on it)
- When force is removed, it retains its original shape
- Hence, we can say that rubber band has fixed shape
- Also, it satisfies all other properties of solids (it has fixed volume, it does not flow like liquids and do not take shape of container)
Question 2
Salt and Sugar do not appear to have fixed shape. Why is it still a solid?
Answer
- Sugar/Salt are made up of tiny crystals
- Shape of these crystals are fixed
- Hence these are called solids
- Sugar/Salt appear to take shape of container where they are kept but this is because of their small size, they do not actually change shape
Properties of Liquids
-
Liquids have
fixed volume, but not fixed shape
They take shape of container they are kept in
-
Particles of Liquids are
closely to each other (but not
as close as solids)
-
Liquid
does not fill container completely like gases
(This is because particles of liquids have higher density than gases and are close to each other)
- Liquids are able to flow easily as particles are able to slide over each other
Properties of Gases
-
Gases have
no fixed volume, no fixed shape
-
Particles of Liquids are
far from each other
-
Gases
fill the container completely
like gases
(This is because particles of liquids have higher density than gases and are close to each other)
-
Gases flow easily
as particles are free to flow easily from one place to another
- Particles of gas can be compressed easily because of their low density