‘Poly' means many
‘Atomic' means atoms
‘Ion' means atom carrying charged
Group of atoms containing charge is called Polyatomic Ion.
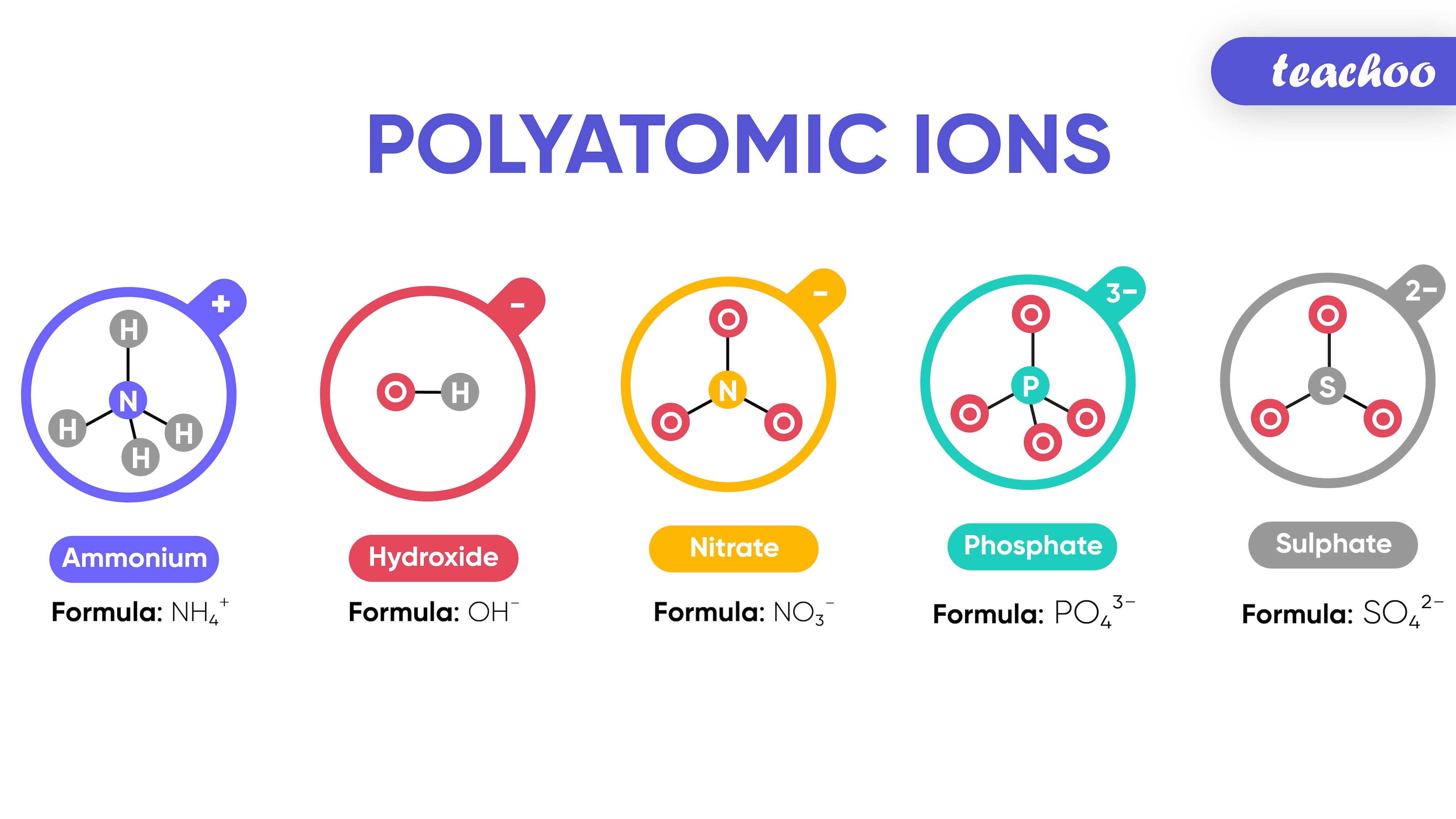
Examples of Polyatomic Ions
- Ammonium - NH 4 +
- Hydroxide - OH -
- Nitrate - NO 3 -
- Phosphate - PO 4 3-
- Sulphate - SO 4 2-
What is a Molecule?
A group of atoms chemically bonded together is Here are the valency of a few elements:
called Molecule.
How to write Formula of compounds formed with Polyatomic Ions
The formula of Polyatomic ion is written in brackets.
Example - In Aluminium Sulphate Al 2 (SO 4 )3
SO
4
2-
is a polyatomic ion consisting of Sulphur (S) and Oxygen (O)
The subscript ‘3’ in Al2(SO
4
)
3
applies to both S and O
And so, SO
4
is put in brackets with subscript ‘3’ outside the brackets.
On the other hand, Al does not have brackets as it is monoatomic.
Example - Magnesium Hydroxide Mg(OH)2
- Valency of Magnesium is 2
- Charge on Magnesium is 2+
- Charge on Hydroxide is 1-
These valencies interchange while writing formula as shown below
What is a Molecule?
A group of atoms chemically bonded together is Here are the valency of a few elements:
called Molecule.
Hence Formula of Magnesium hydroxide is Mg(OH) 2
Note - Hydroxide is written in brackets as 2 is applicable to O and H which means there are 2 OH - ions (2 O and 2 H atoms)
Example - Sodium Hydroxide NaOH
- Valency of Sodium is 1
- Charge on Sodium is 1+
- Charge on Hydroxide is 1-
These valencies interchange while writing formula as shown below
What is a Molecule?
A group of atoms chemically bonded together is Here are the valency of a few elements:
called Molecule.
Hence Formula of Sodium hydroxide is NaOH
