Ethanoic Acid (CH 3 COOH):
- It is also known as acetic acid.
- It is the 2nd member of a homologous series of carboxylic acid.
- Dilute solution of ethanoic acid is called vinegar.

a. Physical Properties:
- It is a colorless liquid having a sour taste and the smell of vinegar.
- It has a higher boiling point as compared to ethanol i.e. 118 o C .
- When ethanoic acid freezes, it forms an ice-like solid. Therefore, it is also known as glacial acetic acid .
- It is also highly soluble in water.
- Since ethanoic acid is acidic in nature, it turns blue litmus red. However, it is a weaker acid as compared to hydrochloric acid (HCl).
b. Chemical properties:
-
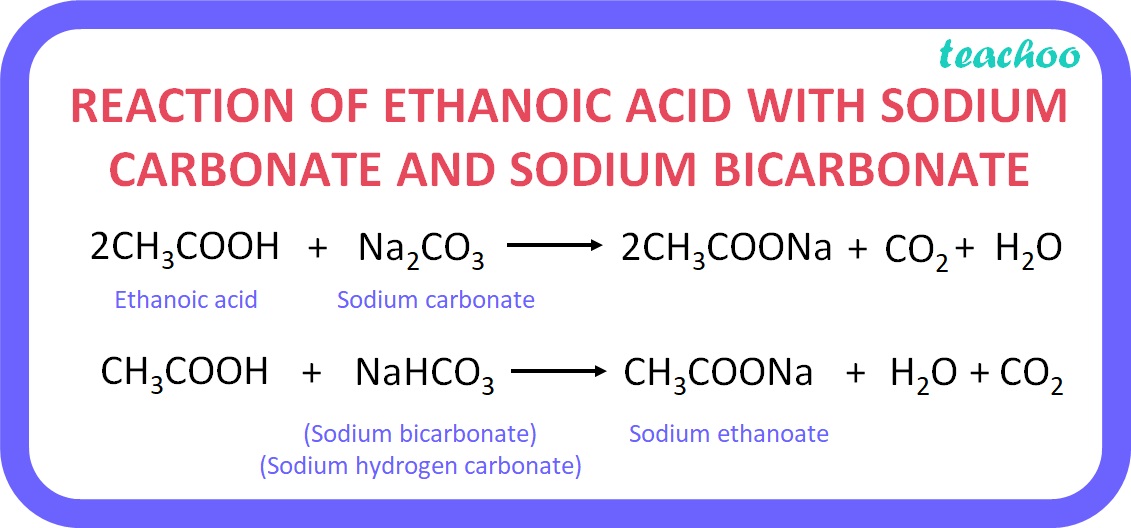
Reaction with carbonates and hydrogen carbonates:
Ethanoic acid reacts with sodium carbonate and sodium hydrogen carbonate to yield salt, water and carbon dioxide gas. The reactions are as follows:
This reaction is used as a test for carboxylic acids because CO 2 gas is released with brisk effervescence and it turns lime water milky.

-
Reaction with sodium hydroxide:
Ethanoic acid reacts with bases to form salt and water.

-
Reaction with Alcohols (Esterification reaction
):
Ethanoic acid reacts with alcohol in the presence of a small amount of concentrated sulphuric acid to form esters which have a fruity smell . Because ester is formed in this reaction, the process is known as esterification.
Hydrolysis of esters: When esters are heated with NaOH the ester breaks down/hydrolyses to give carboxylic acid and alcohol.


Uses of Ethanoic Acid:
- It is used in preparation of dyes, plastics and pharmaceuticals.
- Used for manufacture of acetone and esters used in perfume.
- Vinegar which is diluted ethanoic acid is used in food preparation and preservation.

