Alkenes
It is a hydrocarbon in which carbon atoms are connected by double bonds .
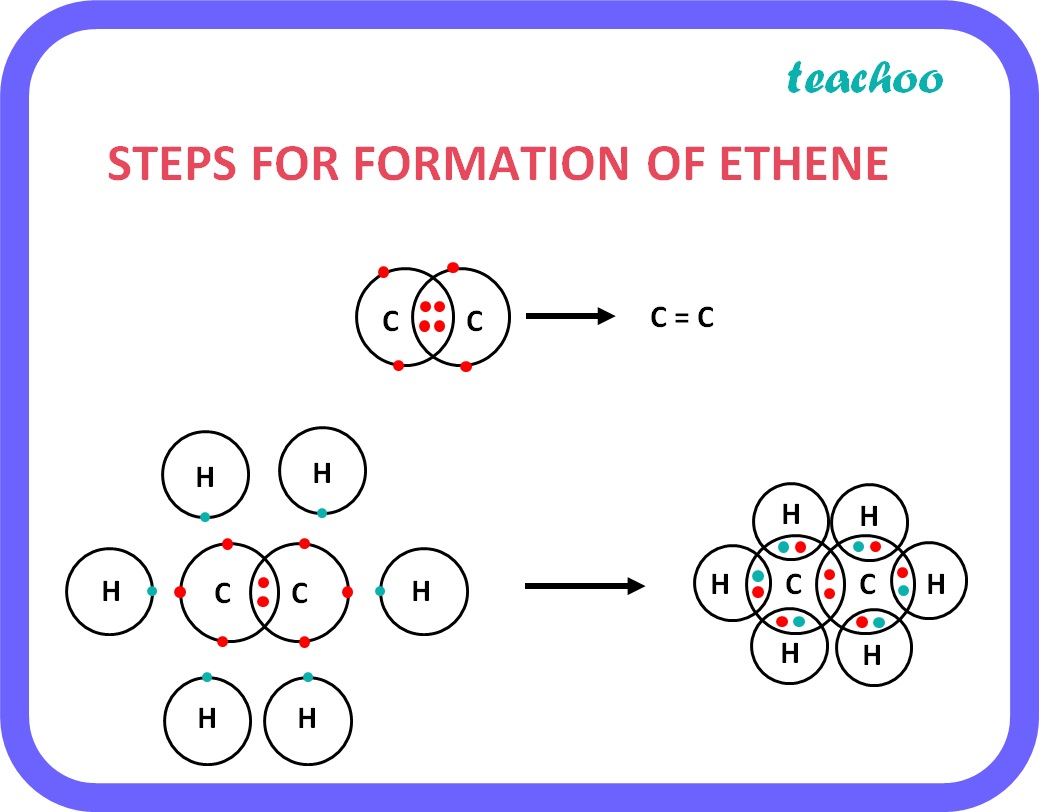
Example: Ethene
- Formula of Ethene is C 2 H 4 .
- There is 1 double bond between 2 Carbon atoms.
- Since Carbon has 4 valence electrons and only 2 of them are shared by other carbon atom, the remaining 2 electrons are shared by 2 hydrogen atoms each.
Hence there are a total of 2 carbon atoms and 4 hydrogen atoms .
General formula for Alkenes
It Is C n H 2n
- Where n is the number of carbon atoms.
|
Number of Carbon Atoms (n) |
n=2 |
n=3 |
n=4 |
|
Name of Alkene |
Ethene |
Propene |
Butene |
|
Structure |

|

|
|
Example: Formula for Butene
- In Propene, there are only 4 carbon atoms.
- So the formula is,
C n H 2n
- Putting n=4
C 4 H 2*4
C 4 H 8

|
Name of Alkene |
Formula Derivation C n H 2n |
Number of Carbon Atoms (n) |
|
Ethene |
C 2 H 4 |
n=2 |
|
Propene |
C 3 H 6 |
n=3 |
|
Butene |
C 4 H 8 |
n=4 |
|
Pentene |
C 5 H 10 |
n=5 |
|
Hexene |
C 6 H 12 |
n=6 |
|
Heptene |
C 7 H 14 |
n=7 |
|
Octene |
C 8 H 16 |
n=8 |
|
Nonene |
C 9 H 18 |
n=9 |
|
Decene |
C 10 H 20 |
n=10 |

