Alkanes
What are Alkanes:
These are the hydrocarbons in which carbon atoms are linked to each other by a single bond.
Example: Methane
- Formula of Methane is CH 4
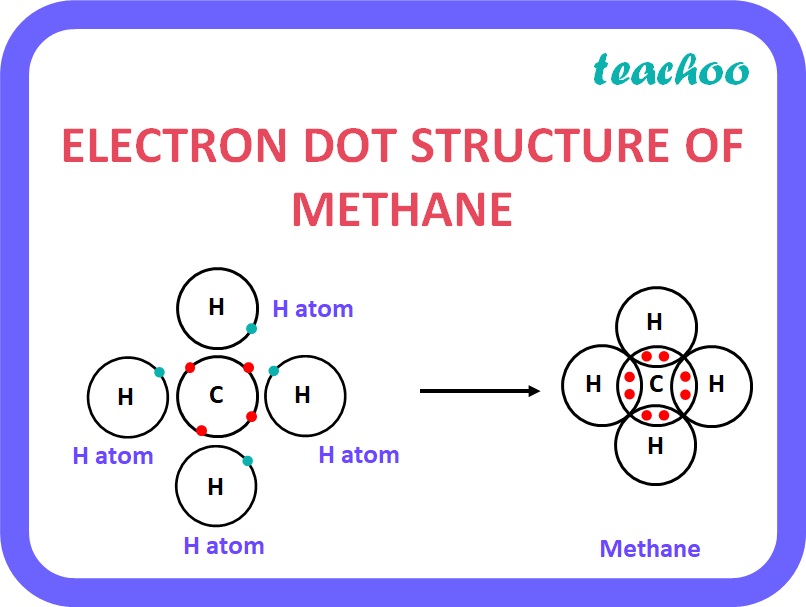
- There is only one Carbon Atom.
- The 1 Carbon atom has 4 Valence electrons .
- It shares these 4 Valence electrons (1 electron each) with 4 Hydrogen Atoms.
A methane molecule has 1 carbon atom and 4 hydrogen atoms .
General formula for Alkanes:
It Is C n H 2n+2
- Where n is the number of carbon atoms.
|
Number of Carbon Atoms (n) |
n=1 |
n=2 |
n=3 |
|
Name of Alkane |
Methane |
Ethane |
Propane |
|
Structure |

|

|
---teachoo.jpg)
|
Propane
- In Propane, there are 3 carbon atoms
- The general formula for alkanes is:
C n H 2n+2
- Putting n=3,
C 3 H 3*2+2
C 3 H 6+2
C 3 H 8
Hence, the formula for propane is, C 3 H 8

|
Name of Alkane |
Formula Derivation C n H 2n+2 |
Number of carbon Atoms |
|
Methane |
CH 4 |
n=1 |
|
Ethane |
C 2 H 6 |
n=2 |
|
Propane |
C 3 H 8 |
n=3 |
|
Butane |
C 4 H 10 |
n=4 |
|
Pentane |
C 5 H 12 |
n=5 |
|
Hexane |
C 6 H 14 |
n=6 |
|
Heptane |
C 7 H 16 |
n=7 |
|
Octane |
C 8 H 18 |
n=8 |
|
Nonane |
C 9 H 20 |
n=9 |
|
Decane |
C 10 H 22 |
n=10 |
